Phosphorus
What is phosphorus?
Phosphorus is a mineral element and a nutient essential for life. It is found in all organic matter, in vegetation as well as in human and animal excreta. It is not toxic, and is naturally present in all aquatic ecosystems. In a young, healthy body of water, phosphorus concentrations are quite low, ensuring normal growth of aquatic plants. In freshwater aquatic ecosystems, phosphorus is a limiting element, meaning that the growth of plants and algae is limited by the availability of phosphorus. The greater the amount of phosphorus in the lake, the more organic material that will be produced. Phosphorus is found in all fertilizers, no matter the type.
Where does phosphorus come from?
The phosphorus found in our aquatic ecosystems comes from two sources, natural and anthropic. Phosphorus found in rock slowly leaches into the hydrologic network. As well, since organic matter contains phosphorus, the decomposition of vegetation, for example tree trunks in the bottom of a waterway, releases a certain amount of phosphorus. The majority of the phosphorus found in aquatic environments does not come from these natural sources. In fact, the massive use of phosphorus in detergents and fertilizers has led to significant exploitation of phosphate ores. By runoff or percolation into the soil, phosphorus introduced into the environment finds its way into our waterways.
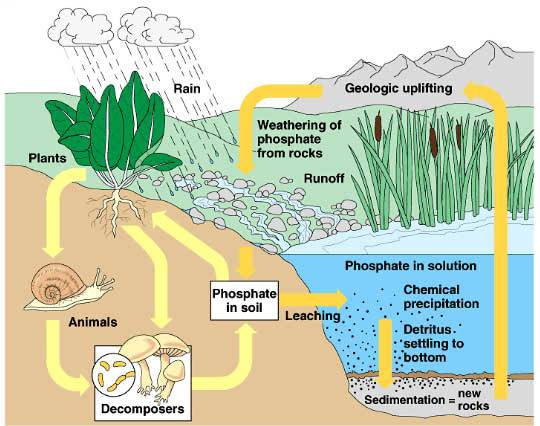
What are the anthropic sources of phosphorus?
The amount of phosphorus in the water in the Lake Memphremagog watershed has increased drastically over the past few decades. This excess phosphorus is the result of the way in which we occupy the territory and the domestic, farming, foresty, industrial and urban activities that we practice, no matter where in the watershed they occur. In fact, though the shores of our lakes and waterways are particularly sensitive, all of the phosphorus released into the environment is likely to end up in the hydrologic network via leaching and runoff.
How does phosphorus make its way into our aquatic ecosystems?
Phosphorus is not particularly soluble, thus it needs a particle to attach to in order to be carried by the movement of water. When it rains, phosphorus attached to a particle will move with the water into the hydrologic network via runoff. If the water enters a tributary, we say that the phosphorus comes from the tributary, otherwise it comes from the surrounding land. We need to keep in mind that all of the phosphorus that is not taken up by terrestrial vegetation will enter the hydrologic network via leaching and runoff, stimulating the growth of aquatic vegetation.
What happens to phosphorus once it enters the lake?
First of all, a lot of the phosphorus that flows towards the lake is consumed by the aquatic vegetation. The rest either flows out of the lake, carried by the current, or else sinks to the bottom. If the lake bottom is well oxygenated, the phosphorus will attach to the sediments and remain trapped there. Sediments can thus accumulate great quantities of phosphorus; this is called the internal phosphorus load. This internal load is normally evacuated during the seasonal turnover of the water, in the case of deep lakes, and during strong wind events in the case of shallow lakes. When sediments are saturated with phosphorus, they cannot absorb more, and the phosphorus remains in the water column, available to be consumed by the aquatic vegetation. As well, the decomposition of vegetable matter whose growth has been stimulated by a strong presence of phosphorus reduces the oxygen concentration near the sediments, creating a vicious cycle. Stirring up sediments, notably by the passage of boats, can also put phosphorus back in suspension in the water column, maiking it available for plant growth.
Why is the presence of high concentrations of phosphorus bad for aquatic ecosystems?
Present in high concentrations, phosphorus leads to excessive plant growth, and can stimulate the proliferation of cyanobacteria. Cyanobacteria represent a serious risk to public health, and the exscessive growth of aquatic vegetation brings with it a plethora of negative consequences, and contributes to the eutrophication of waterways.
What ae the negative impacts of excessive aquatic plant growth?
Excessive growth of aquatic vegetaton leads to the loss of habitat as well as a number of processes that damage the ecosystem.
Modification of ecosystem structure and the loss of biological diversity (loss of habitats)
The presence of excessive concentrations of phosphorus in fresh water ecosystems stimulates the growth of plants and algae, which can modify the structure of the ecosystem, that is to say the space that they occupy, preventing the use of that space by fauna.
Increase in organic matter
Excessive growth of aquatic vegetation increases the volume of organic matter produced by the ecosystem. All of this organic matter eventually finds its way to the bottom, where it is degraded and digested by cocroorganisms.
Depletion of dissolved oxygen (modification and loss of habitat)
To decompose this vegetable matter, microorganisms, like all living things, need oxygen. The decomposition of a large quanitty of dead plant matter absorbs a large quantity of osxygen, which reduces its concentration in the water. Thus, all of the accupants if the ecosystem suffer as a result.
The loss of sportfishing species (loss of use and of recreational potential)
The reduction of oxygen concentrations in water can lead to the axphisiation of fish. The most sensitive species will either leave or die. The majority of sport fish are very sensitive to a lack of oxsygen: trout, bass and pike in particular.
Degradation of swimming areas (loss of use and recreational potential)
Large masses of aquatic plants that develop in swimming zones are not conducive to swimming.
What domestic activities lead to an excessive presence of phosphorus in the hydrologic network?
The use of domestic fertilizers, the use of cleaning products containing phosphorus, and badly maintained septic systems are all potential sources of phosphorus.
The use of fertilizers on residential properties
The use of any type of fertilizer on lawns, flower beds and domestic gardens is a source of phosphorus in the hydrologic network. Each half kilo of fertilizer can stimulate the growth of 500 kilos of aquatic vegetation. Even though they release their nutrients more slowly than chemical fertilizers, natural fertilizers (compost and manure), release just as much phosphorus. So, if the phosphorus is not taken up by terrestrial vegetation before it rains, and is thus carried to the hydrologic network by runoff, it will stimulate aquatic vegetation. The application of fertilizer near a body of water is certainly risky, since the distance to water is shorter, but remember that all of the water in the watershed will eventually find its way to the hydrologic network. The loss of forest cover, large lawn surfaces and the impermeabilization of the soil, all associsated with irresponsible real estate development, are contributing factors to surface runoff. The water that flows on the surface has a greater chance of picking up phosphorus particles and carrying them to the hydrologic network than that that infiltrates the soil. Again, the degradation or absence of a riparian buffer zone is another aggarvating factor, since it is true that dense vegetation near the shoreline can capture and consume a certain proportion of the phosphorus that otherwise would be carried by runoff into the hydrologic network.
Improper maintenance of septic systems
Human excreta are full of phosphorus. A septic system in proper working order retains the majority of this organic matter. On the other hand, a poorly maintained septic system will let the majority of the phosphorus to flow towards the hydrologic hetwork.
The use of household products containing phosphorus
Even today, some household cleaning products, such as laundry and dish detergent, contain a certain amount of phosphorus. This phosphorus flows with the water to the septic system. Conventional septic systems are not designed to eliminate phosphorus suspended in the water. If this phosphorus is not consumed by soil microorganisms, it will eventually find its way to the hydrologic network.
What farming activities promote an excessive presence of phosphorus in aquatic ecosystems?
Spreading fertilizers which do not maximize their absorption by vegetable crops
When fertilizers are not absorbed by cultivated plants, they are carried by water and dump their phosphorus content into the hydrologic network. This is especially true when excessive amounts are spread, when spread too close to waterways, when spread during the plants' dormant period, on impermeable soil, or when the soil is frozen or covered with snow. All agricultural practices that promote runoff are aggravating factors, for example widely spaced plantings, in paritcular corn and soya. Agricultural fertilizers should be spread when conditions favour their uptake by the plantings, and a sufficiently wide buffer zone to filter or absorb all of the phosphorus that is contained in the runoff should be maintained.
Poor containment of animal excreta or waste water
Animal excreta, like all organic matter, is full of phosphorus. Over the past few years, considrable effort has been made to hold these animal excerta in sealed confinement structures, while waiting to be used as fertilizer on agricultural fields, but some small operations, unfortuantely, have not participated in these efforts.
Management of animal excreta via liquid rather than solid manure
Manure and compost release their phosphorus much more slowly than liquid manure or sewage, giving plants more opportunity to absorb the phosphorus before it reaches the hydrologic network.
Insufficient treatment of waste water coming from feed storage infrastructure and exercise yards
Waste water coming from forage storing infrastructure (silos, bunkers, etc.) and exercise yards is loaded with phosphorus. The retention and treatment of this waste water is very often inadequate, as many installations escape the enforcement of standards.
What urban acitivities are likely to send additional quantities of phosphorus into the hydrologic network of Lake Memphremagog?
Overflow from waste treatment plants
Most municipal sewage systems are based on a unitary system principle. That is to say that all waste water, wether from rainfall or sewage systems, are sent via the same pipes to the waste treatment plant. Thus, during heavy rainstorms or snowfalls, the waste trestment plant is overburdened and cannot handle the volume of liquid it receives. The overflow goes directly to aquatic ecosystems. For a long time, we believed in the principle of 'dilution is the solution', but we realize today that frequent overflows are an important source of phosphorus in aquatic ecosystems. In Québec, in 2007 alone, cities have dumped this untreated liquid into waterways, notably in the St-Laurence river, some 45,178 times, because their sewer systems overflowed (L.-J. Francoeur, 2009).
Lack of treatment of rainwater
In cases where rainwater and waste water are not sent to the same pipes, only waste water is sent to the treatment plant, while rainwater is allowed to flow directly into the environment without any treatment whatsoever. This rainwate is loaded with all of the sediments and phosphorus that it picks up while flowing over streets, parking lots and residential lots.
Problems with interconnections between sewage systems
For systems with separate piping, it often happens that an inversion of the connections results in sewage ending up in rainwater systems, daily sending quantities of sewage into the environment without any treatment at all.
Impermeable surfaces
All infrastructure that impermeabilizes the soil promotes surface runoff, which increases the amount of water sent to rainwater collection systems. Large urban centres and parking lots, as well as excessive residential development create impermeable soils.
What forestry activities are likely to send phosphorus into the hydrologic network?
Release of phosphorus contained in the soil
As with all organic matter, forest soils contain important quantities of phosphorus. When these soils are disturbed, for example by the passage of heavy equipment or when the forest cover is removed, they liberate the large quantities of phosphorus that they contain.
Removal of the forest cover
The removal of the forest cover and forest drainage alter the hydrology of the watershed, resulting in more water flowing over the surface and less percolating into the soil. When flowing over the surface, the water is more likely to encounter soil particles to which phosphorus attaches itself, which is then carried to the hydrologic network.
Golf courses: an important source of phosphorus
Massive use of fertilizers
Golf courses use massive quantities of fertilizers as a matter of course to maintain a perfect lawn. As well, the fertilizers used are generally fast phosphorus release types, resulting in its release faster than the plants need. All of the phosphorus not used by the plants goes to sitmulate growth of aquatic plants.
Watering of golf greens
Watering of golf greens promotes the leaching and transport of phosphorus towards the hydrologic network.
Insufficient buffer zones
Even today, many golf courses do not have any riparian buffer zones to absorb a proportion of the phosphorus contained in the runoff from golf greens.
How to limit the flow of phosphorus into the hydrologic network of the Lake Memphremagog watershed?
We can significantly reduce the flow of phosphorus into waterways by changing the way we practice acitivities in our daily lives. To learn how you can contribute to limiting phosphours in the hydrologic network of the Lake Memphremagog watershed, we invite you to refer to the section what can I do? of the web site.
For more information:
RAPPEL, 2008.Technical document on soaps and detergents (in French only)
RAPPEL, 2005 - Phosphorus - a too efficient fertilizer (in French only)
Environment Canada, 2009. Recommendations concerning water quality and phosphorus
IRDA, 2008. Phosphorus mobility - from soil to waterway (in French only)








.png)





.JPG)
































.JPG)























